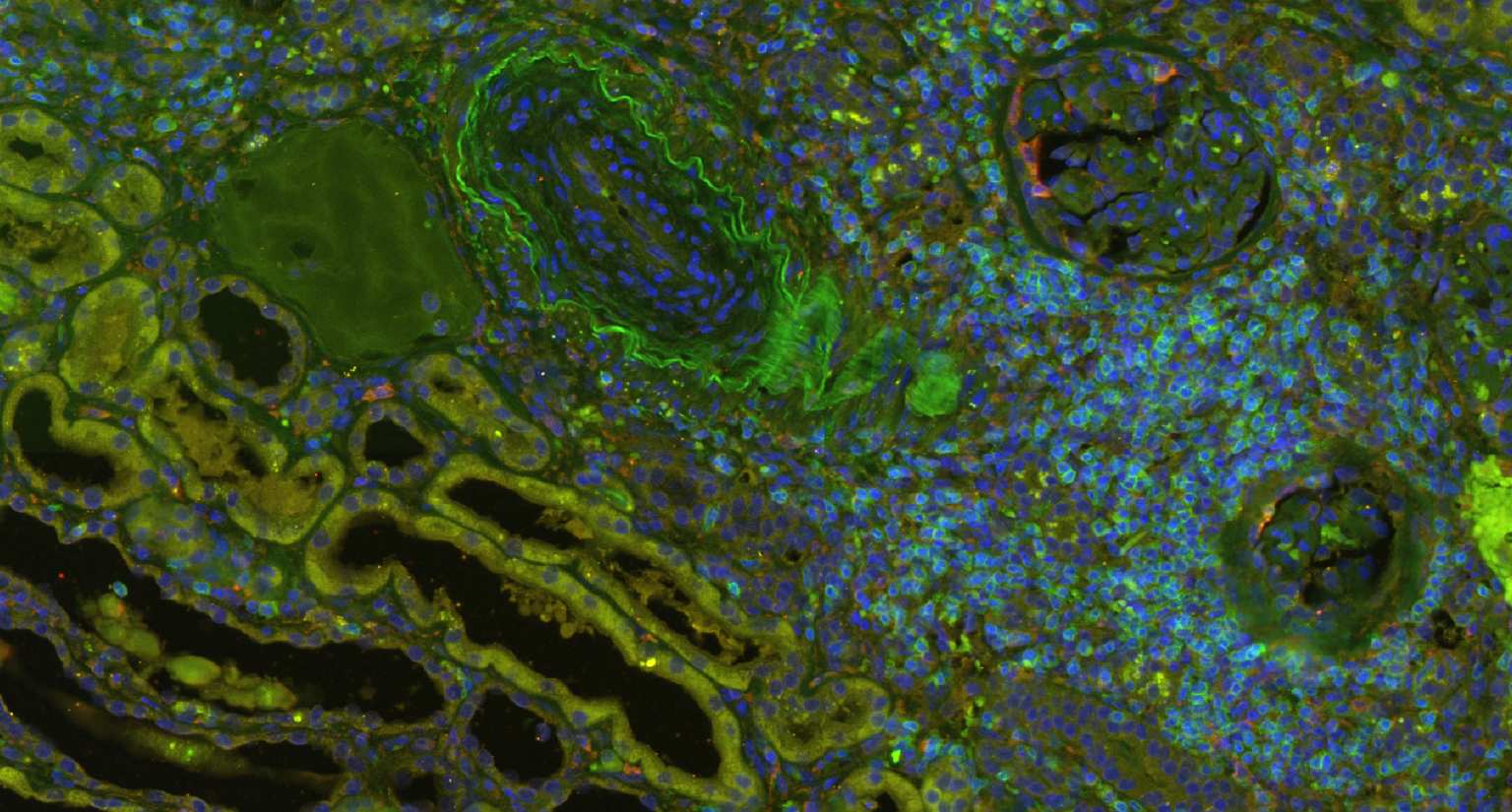
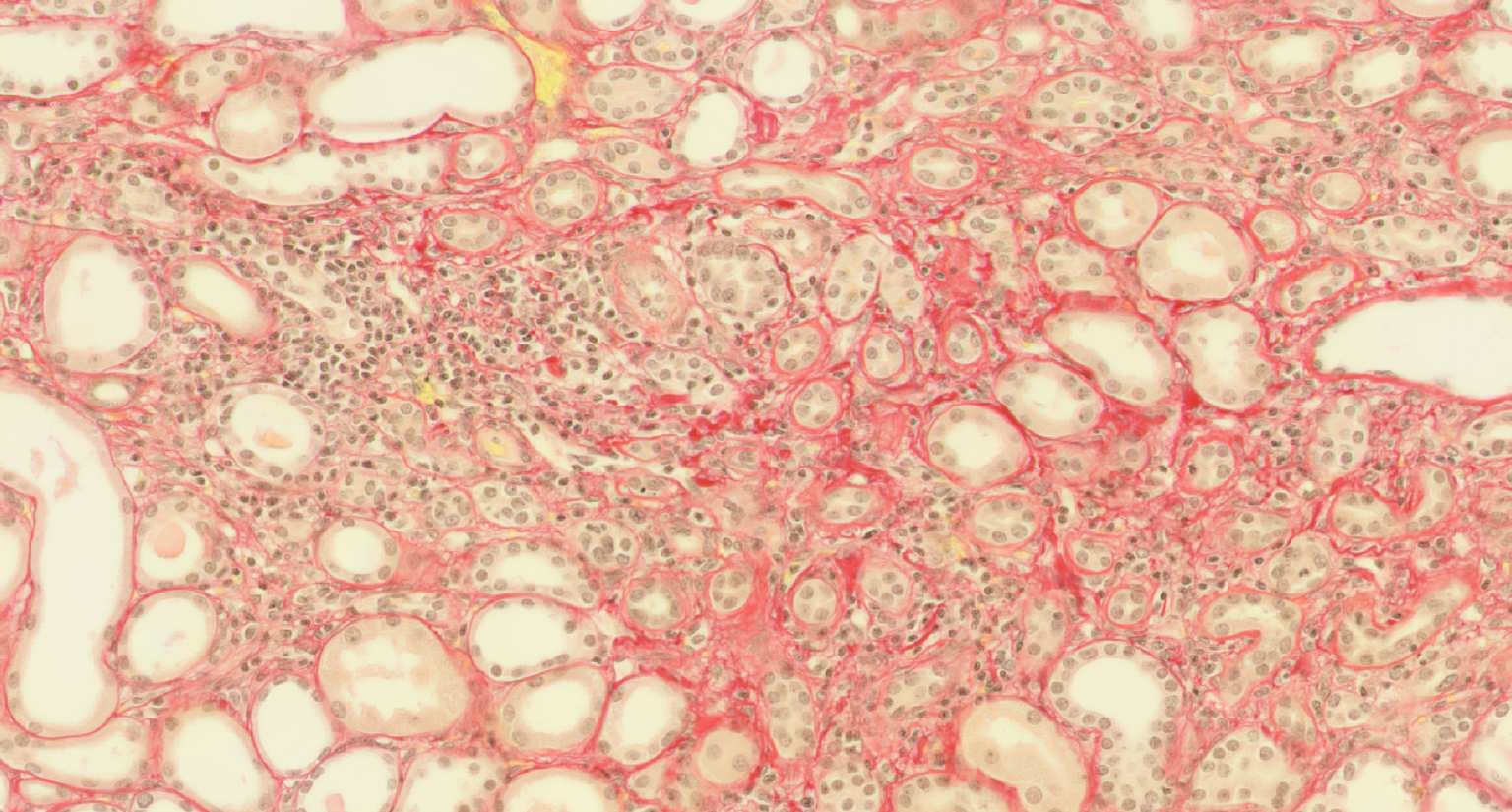
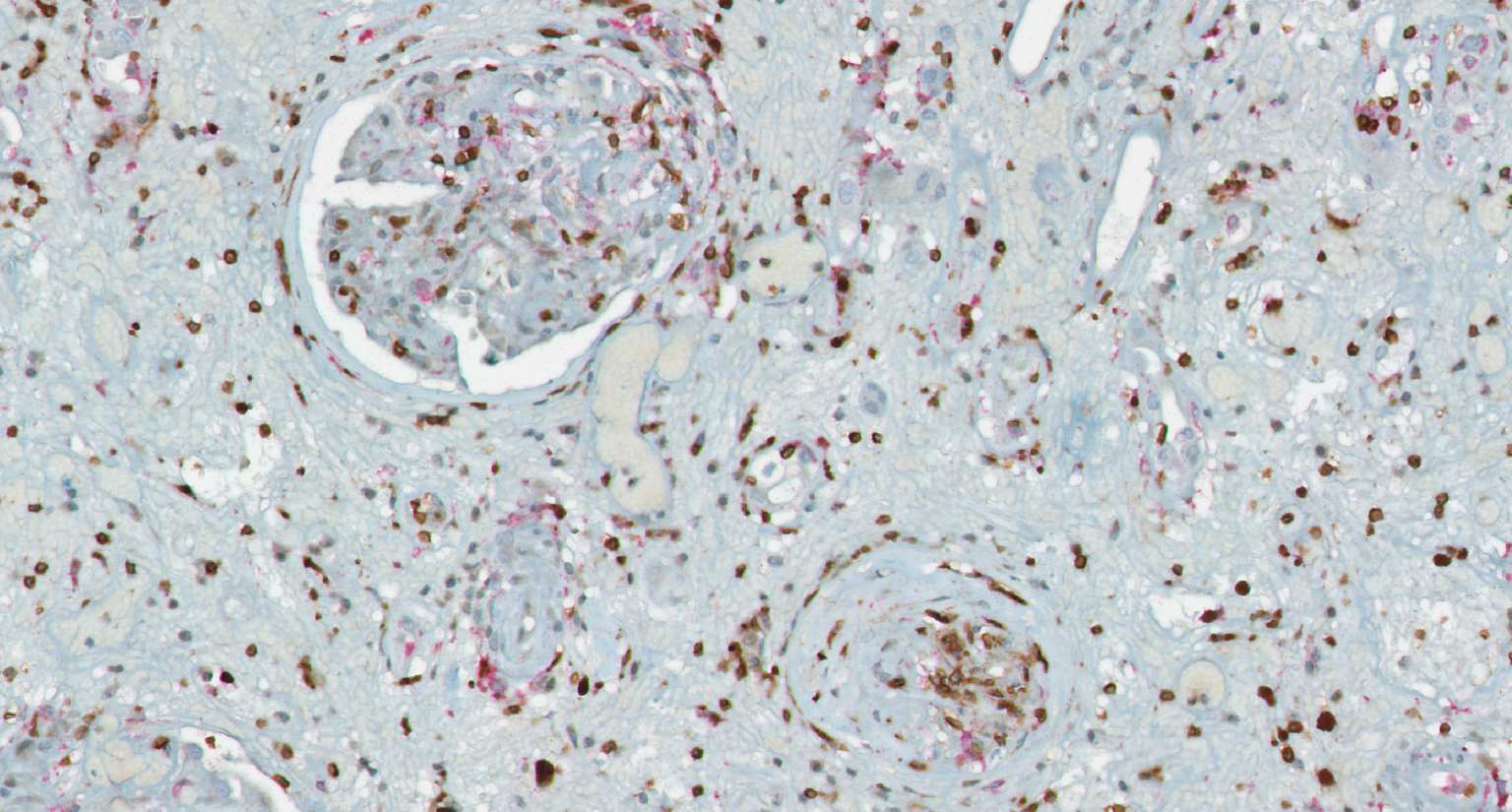
A systems medicine approach to minimize macrophage-associated interstitial fibrosis and tubular atrophy in renal allograft rejection
Transplantation medicine has been very successful in reducing the risk of acute rejection after kidney transplantation. This resulted in better long-term graft survival and shifted the major medical need towards strategies avoiding long-term damage. A slowly progressing process referred to as “Interstitial fibrosis and tubular atrophy (IF/TA)” is currently one of the main causes of functional decline and has become a serious threat to transplanted kidneys. Alternatively activated macrophages, a subgroup of immune cells with multiple functions, e.g., in wound healing and immune regulation to name just two of them, are likely to have a central role for IF/TA. While recent developments in immunotherapy open new paths towards targeting “bad” macrophages while protecting “good” ones, new strategies to guide such therapies are needed. SYSMIFTA applies systems medicine to this field and creates the foundations for personalized therapeutic approaches. The goal is to precisely target those macrophages and their interaction partners that have negative effects on transplanted organs, while preserving the delicate immunological balance required for long-term graft survival. Given the complexity of functional networks in transplant immunology and tissue repair, a systems medicine approach is needed to dissect the complex role of macrophages. The consortium connects mathematicians predicting cell behavior, computer scientists applying satellite image analysis technology to microscopic images of renal biopsies, leading experts for macrophage and lymphocyte biology, and medical experts with outstanding experience in patient care after transplantation. The expected results include dynamic (agent-based) mathematical models reflecting essential mechanisms leading to IF/TA, their calibration and validation with biopsy-based, large-scale clinical data, and the clinical implementation of this systems medicine concept in a knowledge-based, innovative workflows for biopsy evaluation guiding medical decisions. As thousands of patients in Europe still die each year while on the waiting list for transplantation, we consider our project as an important contribution to minimizing loss of transplanted organs by a novel, systems-level approach to predictive, preventive and personalized transplantation medicine.